 par Véto-pharma
par Véto-pharma The health of honeybees lies at the core of the modern beekeeping challenges and is essential for preserving our ecosystems. For more than 30 years, Véto-pharma has been committed to supporting the beekeeping industry with a strong conviction: sustainable beekeeping is only possible through innovation grounded in rigorous scientific research.
This commitment translates into significant and ongoing investments: an experimental apiary of nearly 400 colonies serving as a true open-air laboratory, 10% of the annual revenue dedicated to R&D, and multidisciplinary teams working every day to design, test, and improve solutions for bee health.
But what are the key stages in the development of a bee medicine? Who are the key players involved? What timelines and regulatory requirements must be met before a medicine can be placed on the market? These question, and many more, are answered in our new dedicated video series.
Developing a vet medicine for bees is a long, rigorous, and strictly regulated process. Behind every registered medicine available to beekeepers lies a scientific, technical, and human journey that often remains unknown.
To shed light on this reality, we are making the video series “Behind the scenes of a medicine for bees” accessible to everyone.
Across five episodes, this series explores the scientific, industrial, and regulatory steps required to transform an idea into an authorized veterinary product.
It’s intended for anyone wishing to better understand how veterinary pharmaceutical innovation works when applied to bees.
Contrary to common belief, identifying a promising molecule is only the beginning. Each project requires years of work, up to 12 years, depending on the complexity, along with significant financial investments and a succession of strictly supervised studies. These requirements are essential to ensure the safety of bees, beekeepers, and consumers.
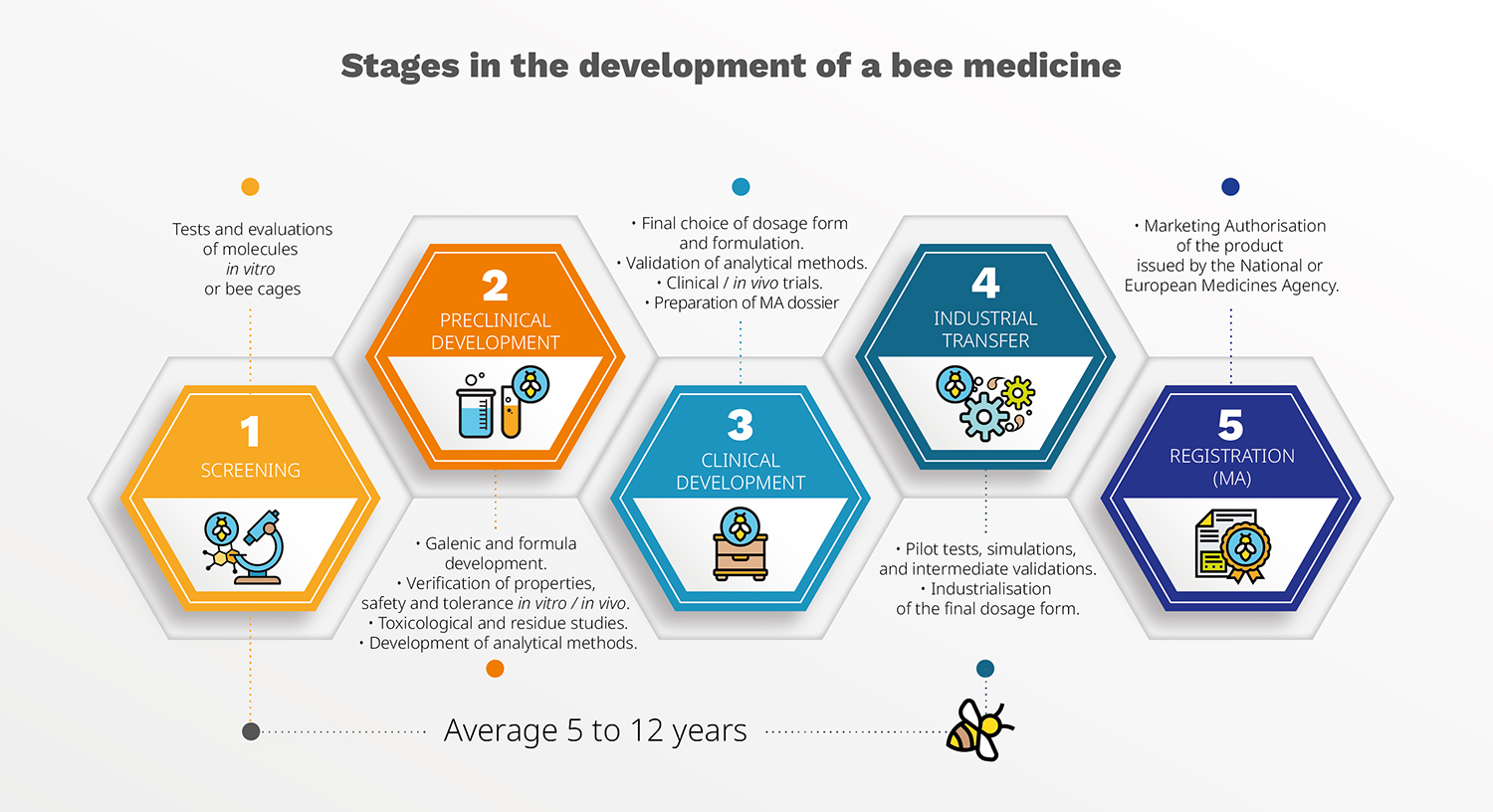
The development of a vet medicine follows a progressive logic, where each step determines the next one and sometimes can even lead back to square one. The series is structured around five key chapters, each commented by Véto-pharma experts directly involved in R&D projects:
Episode 1: Screening stages – Identifying and selecting promising candidates
Commentary by Rémi Padé Bee R&D, Technical and Innovation Manager
Episode 2: Project management and preclinical studies – Organizing, testing, and confirming initial hypotheses
Commentary by Céline Denicourt, Development Department Manager
Episode 3: Clinical Trial Phase – Evaluating efficacy and safety under real-life conditions
Commentary by Rémi Padé Bee R&D, Technical and Innovation Manager
Episode 4: Industrial transfer phase of a medicine – Moving from research to production
Commentary by Romain Fretin, Formulation Technician
Episode 5: Preparing a MA dossier and registration – Obtaining official approval to market a medicine
Commentary by Sandrine Doléans, Director of Regulatory, Quality and Pharmaceutical Affairs
Each video provides additional insight to better understand:
• The sequence of development and their unique challenges,
• Why medicines can take several years before reaching the market?
• How to fit the regulatory requirements governing their use?
• The importance of scientific trials to guarantee safety and efficacy,
• The professions and expertise involved throughout the entire process,
Watch the video: https://youtu.be/6I44EvUa0HM
In this opening episode, Rémi Padé, Bee R&D, Technical and Innovation Manager, introduces the foundation for any development project: the screening phase. This step consists of exploring a large number of possibilities in order to identify candidates with real therapeutic potential.
In practical terms, it involves:

This selection step is essential, as it ensures resources are invested in the most promising projects, while filtering out unsuitable or unsafe candidates early
The development of a medicine is above all a learning journey, in which every result – positive or negative – contributes to scientific progress.
Discover the full series on our YouTube channel.
 by Véto-pharma
by Véto-pharma  by Véto-pharma
by Véto-pharma