Each year, thousands of beekeepers use treatments to combat Varroa destructor. These treatments are based on different active substances (amitraz, oxalic acid, thymol, formic acid, tau-fluvalinate, etc.) and offer different modes of action (evaporation, contact, etc.), kinetics (flash vs. long-term treatments) and application forms (trickling, sublimation, etc.). Yet, it is often forgotten that a mite treatment is, first and foremost, a veterinary medicine.
Honey is a food for human consumption, and consumers must be able to trust that the food they eat has not been exposed to non-authorized molecules or random, estimated dosages. Residues are a real concern, and only registered veterinary medicines can guarantee the compliance with established MRLs (Maximum Residue Levels), as well as compliance with toxity standards for the bees’ and beekeepers’ safety. As a result, veterinary medicines must answer particular constraints and processes inherent to their status. This subject is often overlooked.
A veterinary medicine is a formulation based on an active ingredient that has received a marketing authorisation from the competent authorities (national veterinary agencies and/or the European Medicine Agency [EMA]). Several years pass between the beginning of Research & Development, and the Marketing Authorisation. During this period the Future Marketing Authorisation Holder must complete various steps (search for an active substance, in-vitro tests, clinical data [test in “field” conditions]) to arrive at the marketing authorisation application phase.
To obtain the final authorisation, the future Marketing Autorization Holder must prove the stability, efficacy, and safety (non-harmful effect) of the formulation. A Marketing Authorisation application, including a dossier with numerous research studies, must be submitted. The process of registration is long and costly.
Once these steps have been completed, the national or European agency validates a “SPC” (Summary of Product Characteristics) which summarises all the drug’s characteristics (posology, instructions for use, precautions, secondary effects, etc.) and which also defines its framework of use (in particular posology, precautions, and duration of application).
What about the post-authorisation period?
After authorisation, the medicine may be used but its safety and efficacy must be monitored throughout its use in healthcare practice.
That’s why EU law requires each Marketing Authorisation Holder, National Competent Authority and EMA to operate a pharmacovigilance system through cooperation between the EU Member States, EMA and the European Commission.
First, let’s be clear. Treating against varroa mites with a product that is not a veterinary medicine is illegal. Beekeepers are responsible for the quality of the honey they produce, and the conformity for human consumption. By using a non-registered product:
And the list goes on.
One last critical point is the quality of the product, especially when you buy ready-made products that are not registered (for example, ready-made oxalic strips with glycerol that are currently sold in Eastern Europe). Manufacturing varroa mite treatments is not an easy process and it cannot be done in any random production site. The manufacturing plant has to be GMP certified (Good Manufacturing Practices), which guarantees compliance with European directives and their quality standards. Indeed, it is only possible to manufacture a drug in structures that hold a pharmaceutical manufacturing authorisation. GMP are the principles and guidelines to be followed for manufacturing drugs for human and veterinary use. During manufacturing, the same standards are applied to the drug, whether it is for human or veterinary use.

It means that for every batch that will be released in the market, several controls will be conducted. If there is a deviation, the product will not be sold. This procedure avoids, for example, that a product with a lower quantity of active ingredient would be sold in any given market. When manufacturing a veterinary medicine, the active ingredient concentration is monitored for every batch released.
How will varroa infestations be treated in the future? There are few products currently available that combine a satisfactory level of effectiveness with limited user constraints. But we are aware of the urgent need to develop new active ingredients that will make it possible for varroa to be sustainably controlled, and for treatments to be rotated risk-free.
To achieve this, Véto-pharma has invested in innovation for many years and has created the Véto-pharma’s experimental apiary five years ago, along with a bee laboratory. This laboratory houses one of the company’s major innovation projects: screening new active ingredients to fight varroa. For this purpose, the innovation team developed a unique screening method, which makes it possible to evaluate active ingredients and identify the ones that would be effective against varroa, and safe for the colonies. Not an easy feat! Molecules effective against varroa, whether chemical or organic in origin, are often highly toxic for bees. This is particularly true for many essential oils, which only achieve optimal effectiveness at levels that are very harmful to bees. This is another reason why beekeepers should not test unauthorized products themselves in their hives. Toxicity for honey bees can be very difficult to predict.
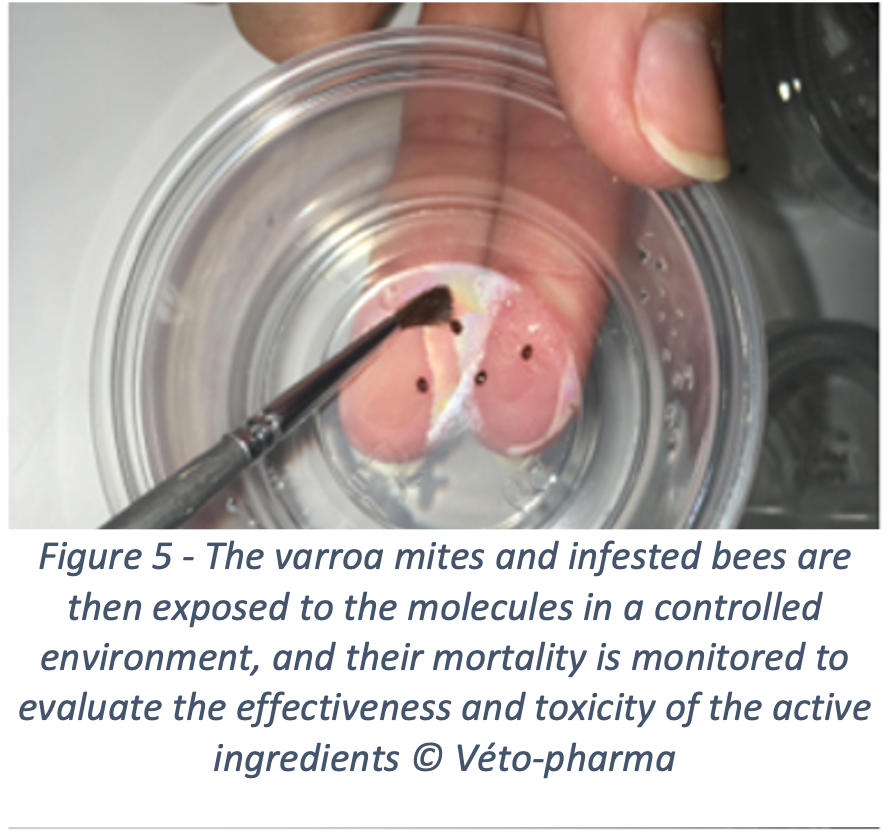
Over the past years, 38 new molecules have been evaluated, some of them showed promising results. These molecules are currently being re-evaluated and will probably lead, if they pass all the tests (efficacy, safety, residues), to the new treatments of tomorrow. By registering new active ingredients, we hope to see a reduction in the use of illegal treatments, thanks to a wider range of products that would be effective, safe, easy to use and accessible.
To learn more about Véto-pharma, please visit www.veto-pharma.eu
1 – Official Journal of the European Union, COMMISSION REGULATION (EU) 2017/623 of 30 March 2017
https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0623&from=PT
Join the Véto-pharma community and receive our quarterly newsletter as well as our occasional beekeeping news. You can unsubscribe at any time if our content does not suit you, and your data will never be transferred to a third party!
© 2019-2025, Véto-pharma. All rights reserved